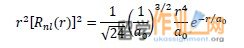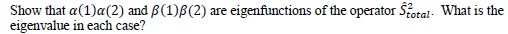|
论文题目:physical chemistry
论文语种:英文
您的研究方向:physical chemistry
是否有数据处理要求:否
您的国家:美国
您的学校背景:要有做题步骤
要求字数:9道题
论文用途:看一下内容
2. Calculate the variance in the radius
3. Calculate the average value of the kinetic and potential energies for the H atom in its groundstate.
4. The force acting between the electron and the proton in the H atom is given by
 Calculate the expectation value[f] for 1s the 2pz and states of Calculate the expectation value[f] for 1s the 2pz and states of the the 
5. Calculate the distance from the nucleus for which the radial probability distribution function for the 2p orbital has its maximum value
6. In this problem, you will calculate the probability density of finding the electron within asphere of radius r for the H atom in its ground state.
(a) Show using integration by parts,
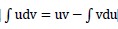 , that , that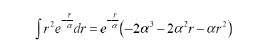
(b) Using this result, show that the probability density of finding the electronwithin a sphere of radius r for the hydrogen atom in its ground state is (setting
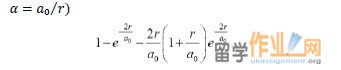
Don’t forget that you still have to integrate over the angles
7. As the principal quantum number n increases, the electron is more likely to be found far fromthe nucleus. It can be shown that for H and for ions with only one electron such as He+.
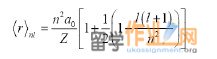
Calculate the value of n for an s state in the hydrogen atom such that r = 1000 a0. Round upto the nearest integer.
8. Using the result of Problem 6, calculate the probability of finding the electron in the 1s state outside a sphere of radius

9. Classify the following functions as symmetric, antisymmetric, or neither in the exchange of electrons 1 and 2:
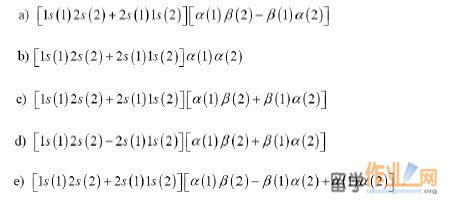
10. The operator for the square of the total spin of two electrons is
|
 |
|||
| 网站地图 |
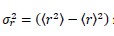 if the H atom wave function is
if the H atom wave function is